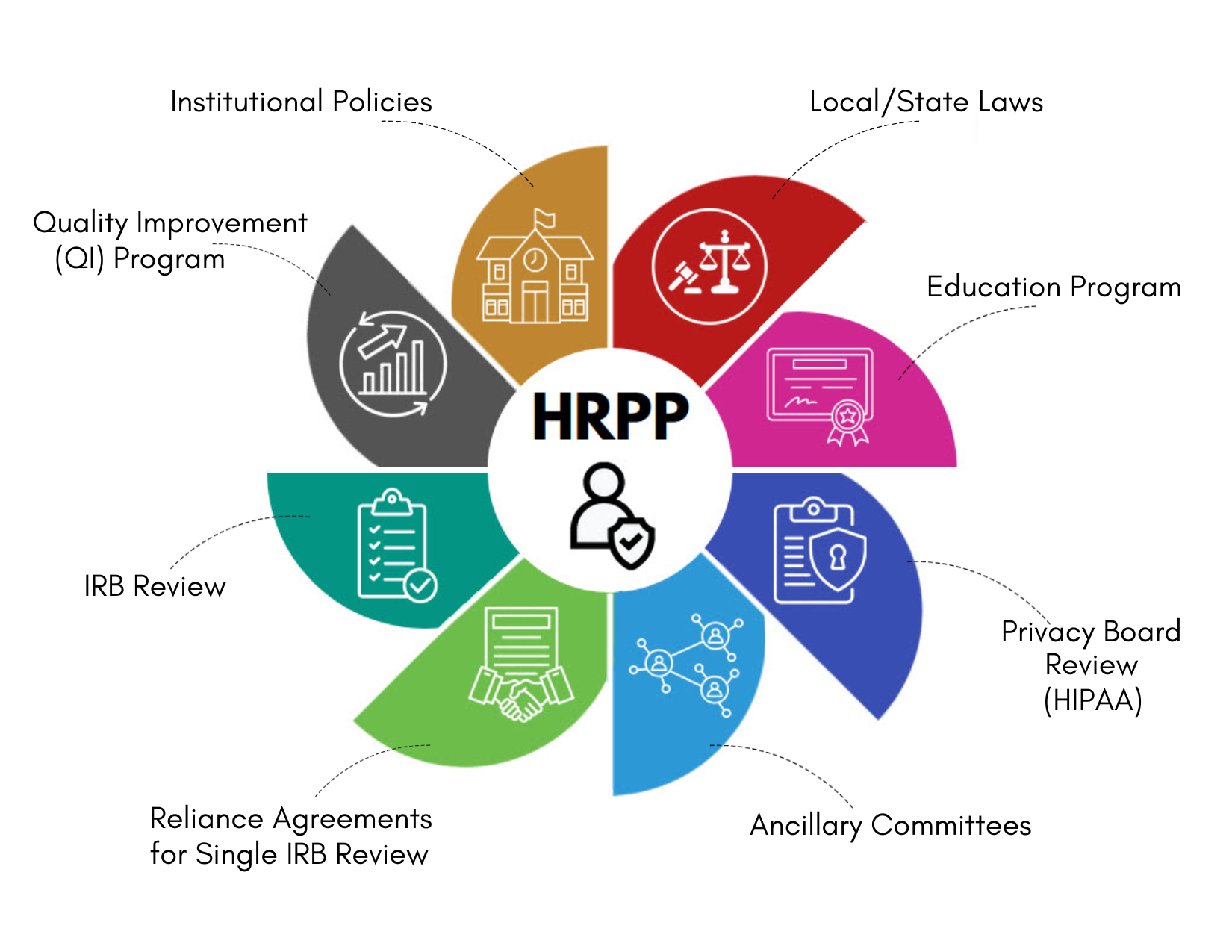
Institutional Policies: The HRPP maintains standard operating procedures (SOPs) that apply to all campuses for research involving human participants.
Local/State Laws: The HRPP verifies compliance with local and state laws that pertain to human participant research for the IRBs and researchers.
Education Program: The education program oversees training requirements for all individuals engaged in human participant research. Additional training and education opportunities are also available and may be requested by individuals or groups.
Quality Improvement (QI) Program: The QI program monitors the effectiveness of all research activities through routines, for-cause, internal, and self-assessment evaluations to ensure compliance with federal regulations, state laws, and OU policies and procedures.
IRB Review: The HRPP oversees two IRB Offices: HSC and Norman. The IRBs review all human participant research protocols in accordance with applicable federal regulations, state laws, and OU policies and procedures to ensure the rights and welfare of a people involved in research are protected.
Reliance Agreements / Single IRB (sIRB) Review: The HRPP facilitates and executes reliance agreements with outside institutions when a single IRB will review and provide oversight for multi-site, collaborative research. Reliance agreements are executed when OU will rely on an outside IRB and when an OU IRB will serve as the reviewing, single IRB for outside sites.
Privacy Board Review (HIPAA): The IRB serves as the Privacy Board for the University. In addition to approving requests for waivers of authorization, the Privacy Board reviews research activities that involve protected health information (PHI) even when the research does not meet the definition of human participant research.
Ancillary Committees: Ancillary Committees includes but are not limited to: Institutional Biosafety Committee (IBC), Radiation Safety Committee (RSC), Conflicts of Interest Committee (COI), and SCC Protocol Review and Monitoring Committee (PRMC). The IRBs will not grant final approval of protocols until all ancillary committee reviews applicable to the research are completed.